UNIK
Being the first synthetic biology research undertaking of its magnitude in Europe, the UNIK Synthetic Biology initiative has paved the way for synthetic biology as a research field to be successfully established at University of Copenhagen. Due to its crossdisciplinary approach, UNIK Synthetic Biology involves a diverse set of research projects aiming at producing e.g. biochemicals, energy, personalized medicine, biomaterials, and bionanoelectronics for novel diagnostic and biosensing tools.

Crossdisciplinary Workgroups
The participating scientific research groups take their starting point in studying biological membranes and in particular the membrane bound proteins that are responsible for the essential processes of life: from photosynthesis in plants to the transportation of dopamine across membranes in the brain. In addition to the health, natural, and life sciences research conducted within UNIK Synthetic Biology, an important part of the UNIK project is to clarify the philosophical and ethical aspects of synthetic biology.
The participants in the UNIK initiative are involved in one of the crossdisciplinary collaborative workgroups, on which the crossdisciplinary research is based. Read more about these below.
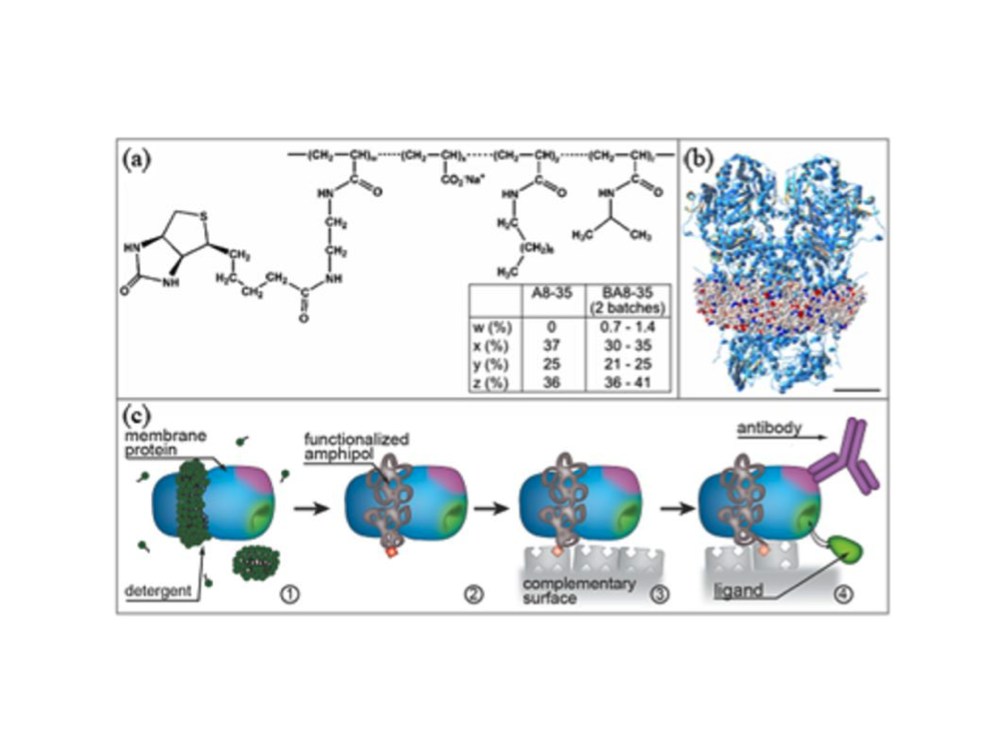
The researchers at Center for Synthetic Biology work in many different directions. But whether they are investigating a potential drug target receptor, a transporter protein for a novel vesicle design, or an enzyme in a promising biochemical synthesis pathway, many of the researchers are working with membrane bound proteins. The Protein Reconstitution and Expresssion workgroup comprises an interdisciplinary team of pharmacologists, biophysicists, and biochemists that are focusing on the preparation of membrane proteins for future structural and functional studies. The unifying methodological approach is based on the expression, purification and reconstitution of selected protein complexes and their components into lipid vesicles, amphipols or nanodiscs. Examples of membrane proteins in focus are ion channels, G protein-coupled receptors, ATP-driven transmembrane pumps including their subunits, complexes of specific cytochrome P450 monooxygenases, reductases, glucosyltransferases, and photosystem I. One of the objectives of the workgroup is to develop methods for expressing and reconstituting novel protein complexes. These methods are expected to be key in developing new technologies based on synthetic biology.
Workgroup Participants
Thomas Günther-Pomorski (Chair of workgroup)
Karen Martinez
Peter Naur
Lisa Theorin
Knud J. Jensen
Søren Roi Midtgaard
Bo Højen Justesen
Selma Maric
Workgroup Publications List
Marek, M., Milles, S., Schreiber, G., Daleke, D.L., Dittmar, G., Herrmann, A., Müller, P., Pomorski, T.G. The yeast plasma membrane ABC transporter Aus1: Purification, characterization and effect of lipids on its activity. J. Biol. Chem. 286: 21835-21843 (2011) I.F. 5.328
'
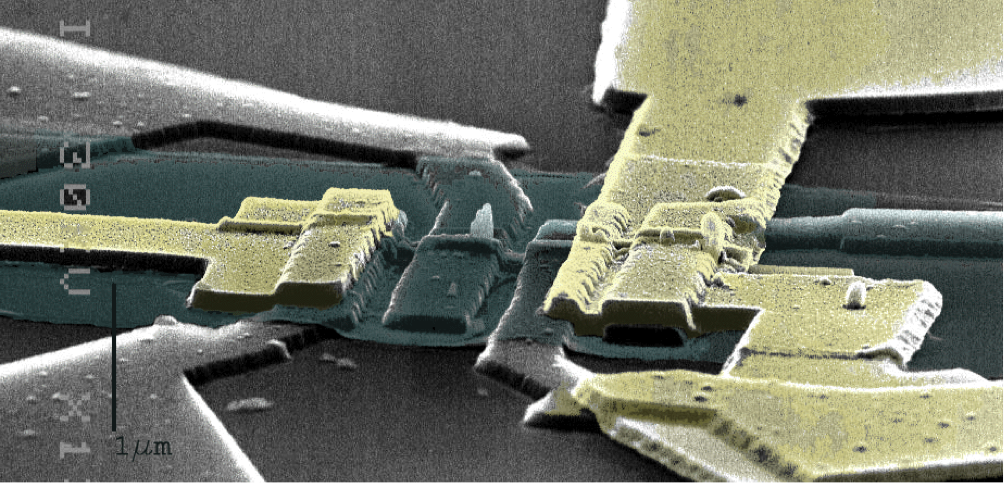
Nanotechnology provides nanomaterials such as nanowires, carbon nanotubes, and graphene and enables fabrication of nanostructured surfaces and functional devices. When combined with electronics, the intrinsic properties of the new materials can be exploited for highly sensitive detection and integrated parallelization, for instance in arrays of nanosensors.
Bionanoelectronics is an emerging interdisciplinary field dealing with the interface of biological samples with nanomaterials, thanks to their size compatibility. The superior features of nanomaterials are used for the development of novel biosensors of high sensitivity. Numerous medical applications will result from this novel generation of biosensors, notably in diagnostics and in drug discovery.
The Nanoscience Center and the UNIK Synthetic Biology programme of University of Copenhagen initiated activities in these areas several years back via interdisciplinary collaborations between groups Nanophysics, Bionanotechnology, and Chemistry. The focus has been on semiconductor nanowires and both electrical and optical detection schemes are investigated.
The activities cover:
- Label-free biosensors based on nanowire field effect transistors (Bio-FET). These devices convert biological events (e.g. ligand–protein intractions, protein–protein interactions) into electrical signals. They will be used for the detection of low concentrations of molecules and proteins in liquid. These biosensors will be suitable for the detection of biomarkers, traditionnally used as indicators of diseases.
- Arrays of vertically aligned nanowires as cellular probes with limited invasiveness. In the initial stages of the hunt for new drugs, basic tests are run in which individual cells are exposed to a drug candidate, and the reaction of each cell is monitored using optical techniques. The cell response will be monitored using this new biochip after stimulation by drug candidates. In this way, our researchers can contribute to the development of tools suitable for the discovery of new drugs.
Workgroup Participants
Karen Martinez (Chair of workgroup)
Jesper Nygård
Katrine Rostgaard
Jan H. Jensen
Martin Hediger
Nina Buch-Månson
Noémie Lloret
Sara Bonde
Thor Møller
Trine Berthing
Volker Wirth
Workgroup Publications List
Hediger, M.R., Jensen, J.H. and De Vico, L.: BioFET-SIM Web Interface: Implementation and Two Applications. PLoS ONE (2012) Submitted
Berthing, T., Bonde, S., Sørensen, C.B, Utko, P., Nygård, J. and Martinez, K.L. 'Intact Mammalian Cell Function on Semiconductor Nanowire Arrays: New Perspectives for Cell-Based Biosensing' Small 7 (5) 640–647 (2011) https://onlinelibrary.wiley.com/doi/10.1002/smll.201001642/abstract
Nanowires (NWs) are attracting more and more interest due to their potential cellular applications, such as delivery of compounds or sensing platforms. Arrays of vertical indium-arsenide (InAs) NWs are interfaced with human embryonic kidney cells and rat embryonic dorsal root ganglion neurons. A selection of critical cell functions and pathways are shown not to be impaired, including cell adhesion, membrane integrity, intracellular enzyme activity, DNA uptake, cytosolic and membrane protein expression, and the neuronal maturation pathway. The results demonstrate the low invasiveness of InAs NW arrays, which, combined with the unique physical properties of InAs, open up their potential for cellular investigations.
De Vico,L., Sørensen, L.H., Iversen,L., Rogers,D.M., Sørensen,B.S., Brandbyge,M., Nygaard,J., Martinez,K.L. and Jensen,J.H. Quantifying signal changes in nano-wire based biosensors Nanoscale 3:706-717 (2011)
In this work we present a computational methodology for predicting the change in signal (conductance sensitivity) of a nano BIOFET sensor (a sensor based on a biomolecule binding another biomolecule attached to a nano-wire field effect transistor) upon binding its target molecule. The methodology is a combination of the screening model of surface charge sensors in liquids developed by Brandbyge and co-workers [Sørensen et al., Appl. Phys. Lett. 2007, 91, 102105.], with the PROPKA method for predicting the pH-dependent charge of proteins and protein-ligand complexes, developed by Jensen and co-workers [Li et al., Proteins Struct. Funct. Bioinf. 2005, 61, 704-721, Bas et al., Proteins Struct. Funct. Bioinf. 2008, 73, 765-783]. The predicted change in conductance sensitivity based on this methodology is compared to previously published data on nano BIOFET sensors obtained by other groups. In addition, the conductance sensitivity dependence from various parameters is explored for a standard wire, representative of a typical experimental setup. In general, the experimental data can be reproduced with sufficient accuracy to help interpret them. The method has the potential for even more quantitative predictions when key experimental parameters (such as the charge carrier density of the nano-wire or receptor density on the device surface) can be determined (and reported) more accurately.
De Vico, L., Iversen,L., Sørensen,M.H., Brandbyge.M., Nygaard,J., Martinez,K.L and and Jensen,J.H. Predicting and rationalizing the effect of surface charge distribution and orientation on nano-wire based FET bio-sensors Nanoscale 3:3635-3640 (2011)
A single charge screening model of surface charge sensors in liquids (De Vico et al.,Nanoscale, 2011, 3, 706–717) is extended to multiple charges to model the effect of the charge distributions of analyte proteins on FET sensor response. With this model we show that counter-intuitive signal changes (e.g. a positive signal change due to a net positive protein binding to a p-type conductor) can occur for certain combinations of charge distributions and Debye lengths. The new method is applied to interpret published experimental data on Streptavidin (Ishikawa et al., ACS Nano, 2009, 3, 3969–3976) and Nucleocapsid protein (Ishikawa et al., ACS Nano, 2009, 3, 1219–1224).
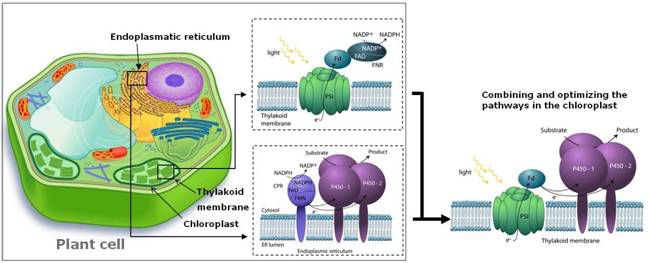
“The Lightdriven Biosynthesis workgroup has a strong expertise within photosynthesis focusing on photosystem I (PSI) and chloroplast biology. One of our main focus points is manipulation of the photosynthetic chloroplasts, which provides a platform to engineer new pathways and establish bio-factories synthesizing desired industry products for pharmaceuticals, fine chemicals, fibres or fuels. Especially terpenoids are of interest due to their pharmacological properties, including anti-cancer activity. However, their availability is generally very limited and organic synthesis is currently not feasible. By re-routing biosynthetic pathways and optimizing the chain of energy transfer we can overcome the inherent limitations in plants to channel photosynthetically fixed carbon and the light-excited electrons directly into production of such desired bioactive natural products.
Our knowledge of PSI and chloroplasts is combined with structural information and topology gained from small angle X-ray scattering (SAXS) and atomic force microscopy (AFM) in the group of Kell Mortensen and Lise Arleth from the Department of Basic Sciences and Environment. Furthermore, biophysics is applied to characterize the electron transport chains and the possibilities of immobilization of the PSI complex. In collaboration with Jesper Nygård from the Niels Bohr Institute a project has been initiated where nanowires are attached directly to PSI to produce a full nano-circuit, which has potential in the development of nano-sensors. We are thus gaining many advantages from the cross-disciplinary research network within the Center.
Our workgroup has identified genes for several of the 23 subunits of the PSI holocomplex and has elucidated the function of these subunits at the molecular and physiological level. Transgenic plants lacking individual subunits constitute important experimental tools. Current topics include structure, function and assembly of the PSI complex and engineered mechanisms to protect the complex from photodestruction when plants are subjected to high solar radiation or nutrient deficiency. Besides nuclear and chloroplast transformation and a host of biochemical tools, fast optic methods have been established to measure photosynthetic partial reactions.”
- Poul Erik Jensen, Chair of Workgroup.
Workgroup Participants
Poul Erik Jensen
Peter Naur
Birger Lindberg Møller
Knud J. Jensen
Agnieszka Zygadlo Nielsen
Lærke Marie Münter Lassen
Thiyagarajan Gnanasekaran
Natascha Kristine Krahl Hansen
Søren Bak
Bibi Emilie Friis Ziersen
Workgroup Publications
Jensen, K., Johnston, J.B, Ortiz de Montellano, P.R. and Møller, B.L. Photosystem I from plants as a bacterial cytochrome P450 surrogate electron donor: terminal hydroxylation of branched hydrocarbon chains Biotechnology Letters 34:239–245 (2012)
The ability of cytochrome P450 enzymes to catalyze highly regio- and stereospecific hydroxylations makes them attractive alternatives to approaches based on chemical synthesis but they require expensive cofactors, e.g. NAD(P)H, which limits their commercial potential. Ferredoxin (Fdx) is a multifunctional electron carrier that in plants accepts electrons from photosystem I (PSI) and facilitates photoreduction of NADP? toNADPH mediated by ferredoxin-NAD(P)H oxidoreductase (FdR). In bacteria, the electron flow is reversed and Fdx accepts electrons from NADPH via FdR and serves as the direct electron donor to bacterial P450s. By combining the two systems, we demonstrate that irradiation of PSI can drive the activity of a bacterial P450, CYP124 from Mycobacterium tuberculosis. The substitution of the costly cofactor NADPH with sunlight illustrates the potential of the light-driven hydroxylation system for biotechnology applications.
Jensen K., Jensen P.E. and Møller B.L. Light-driven chemical synthesis Trends in Plant Science 17 (2) (2012)
Depletion of the fossil fuel reserves of the Earth has prompted research into sources of renewable and sustainable energy, and feedstock for the chemical and pharmaceutical industries to support the transition towards a bio-based society. Photosynthesis efficiently captures solar energy, but its subsequent conversion into chemical energy in the form of biomass is limited to a final output in the 1–4% range. Re-routing of photosynthetic electron transport and reducing power directly into desired biosynthetic pathways offers a new avenue for sustainable production of high-value products.
Bjerg-Jensen, N., Zagrobelny, M., Hjernø, K., Olsen, C.E., Houghton-Larsen, J., Borch, J., Møller, B.L. and Bak, S. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nature Communications 2:273 (2011)
For more than 420 million years, plants, insects and their predators have co-evolved based on a chemical arms race including deployment of refined chemical defence systems by each player. Cyanogenic glucosides are produced by numerous plants and by some specialized insects and serve an important role as defence compounds in these intimate interactions. Burnet moth larvae are able to sequester cyanogenic glucosides from their food plant as well as to carry out de novo biosynthesis. Here we show that three genes (CYP405A2, CYP332A3 and UGT33A1) encode the entire biosynthetic pathway of cyanogenic glucosides in the Burnet moth Zygaena filipendulae. In both plants and insects, convergent evolution has led to two multifunctional P450 enzymes each catalysing unusual reactions and a glucosyl-transferase acting in sequence to catalyse cyanogenic glucoside formation. Thus, plants and insects have independently found a way to package a cyanide time bomb to fend off herbivores and predators
Jensen K., Jensen P.E. and Møller B.L. 'Light-Driven Cytochrome P450 Hydroxylations.' ACS Chemical Biology 6 (6), 533–539 (2011)
Plants are light-driven “green” factories able to synthesize more than 200,000 different bioactive natural products, many of which are high-value products used as drugs (e.g., artemisinin, taxol, and thapsigargin). In the formation of natural products, cytochrome P450 (P450) monooxygenases play a key role in catalyzing regio- and stereospecific hydroxylations that are often difficult to achieve using the approaches of chemical synthesis. P450-catalyzed monooxygenations are dependent on electron donation typically from NADPH catalyzed by NADPH-cytochrome P450 oxidoreductase (CPR). The consumption of the costly cofactor NADPH constitutes an economical obstacle for biotechnological in vitro applications of P450s. This bottleneck has been overcome by the design of an in vitro system able to carry out light-driven P450 hydroxylations using photosystem I (PSI) for light harvesting and generation of reducing equivalents necessary to drive the P450 catalytic cycle. The in vitro system is based on the use of isolated PSI and P450 membrane complexes using ferredoxin as an electron carrier. The turnover rate of the P450 in the light-driven system was 413 min−1 compared to 228 min−1 in the native CPR-catalyzed system. The use of light as a substitute for costly NADPH offers a new avenue for P450-mediated synthesis of complex bioactive natural products using in vitro synthetic biology approaches.
Jensen, K., Osmani, S.A, Hamann, T., Naur, P. and Møller, B.L. Homology modeling of the three membrane proteins of the dhurrin metabolon: Catalytic sites, membrane surface association and protein–protein interactions. Phytochemistry 71: 2113-2123 (2011)
Formation of metabolons (macromolecular enzyme complexes) facilitates the channelling of substrates in biosynthetic pathways. Metabolon formation is a dynamic process in which transient structures mediated by weak protein–protein interactions are formed. In Sorghum, the cyanogenic glucoside dhurrin is derived from L-tyrosine in a pathway involving the two cytochromes P450 (CYPs) CYP79A1 and CYP71E1, a glucosyltransferase (UGT85B1), and the redox partner NADPH-dependent cytochrome P450 reductase (CPR). Experimental evidence suggests that the enzymes of this pathway form a metabolon. Homology modeling of the three membrane bound proteins was carried out using the Sybyl software and available relevant crystal structures. Residues involved in tight positioning of the substrates and intermediates in the active sites of CYP79A1 and CYP71E1 were identified. In both CYPs, hydrophobic surface domains close to the N-terminal trans-membrane anchor and between the F0 and G helices were identified as involved in membrane anchoring. The proximal surface of both CYPs showed positively charged patches complementary to a negatively charged bulge on CPR carrying the FMN domain. A patch of surface exposed, positively charged amino acid residues positioned on the opposite face of the membrane anchor was identified in CYP71E1 and might be involved in binding UGT85B1 via a hypervariable negatively charged loop in this protein.
Jørgensen, K., Morant, A.V., Morant, M., Jensen, N.B., Olsen, C.E., Kannangara, R., Motawia, M.S., Moller, B.L. and Bak, S. Biosynthesis of the Cyanogenic Glucosides Linamarin and Lotaustralin in Cassava: Isolation, Biochemical Characterization, and Expression Pattern of CYP71E7, the Oxime-Metabolizing Cytochrome P450 Enzyme. Plant Physiology 155(1):282-292 (2011)
Cassava (Manihot esculenta Crantz) is a eudicotyledonous plant, which produces the valine- and isoleucine-derived cyanogenic glucosides lotaustralin and linamarin with the corresponding oximes and cyanohydrins as key intermediates. CYP79 enzymes catalyzing amino acid to oxime conversion in cyanogenic glucoside biosynthesis are known from several plants including M. esculenta. The enzyme system converting oxime into cyanohydrin has previously only been identified in the monocotyledonous plant Sorghum bicolor Moench. Using this S. bicolor CYP71E1 sequence as a query in a BLASTp search, a putative functional homologue which exhibited a ~50% amino acid sequence identity was found in M. esculenta. The corresponding full length cDNA clone was obtained from a plasmid library prepared from M. esculenta shoot tips and was assigned CYP71E7. Heterologous expression of CYP71E7 in yeast afforded microsomes converting 2-methylbutanal oxime (isoleucine-derived oxime) and 2-methylpropanal oxime (valine-derived oxime) to the corresponding cyanohydrins which dissociate into hydrogen cyanide and 2-butanone and acetone, respectively. The volatile ketones were detected as 2.4-dinitrophenylhydrazone derivatives by LC-MS. A KS of ~0.9 μM was determined for 2-methylbutanal oxime based on substrate binding spectra. CYP71E7 exhibits low specificity for the side chain of the substrate and catalyzes conversion of aliphatic and aromatic oximes with turnovers of ~17, 21, 8 and 1 min-1 for the oximes derived from isoleucine, valine, tyrosine and phenylalanine, respectively. In tube in situ PCR showed that in nearly unfolded leaves, CYP71E7 is preferentially expressed in specific cells in the endodermis and in most cells in the first cortex cell layer. In fully unfolded leaves, the expression is pronounced in the cortex cell layer just beside the epidermis and in specific cells in the vascular tissue cortex cells. Thus the CYP71E7 transcript co-localizes with CYP79D1 and CYP79D2. We conclude that CYP71E7 is the oxime-metabolizing enzyme in cyanogenic glucoside biosynthesis in M. esculenta.
Kannangara, R., Motawia, M.S., Hansen, N.K.K, Paquette, S.M., Olsen, C.E., Møller, B.L. and Jørgensen, K. Characterization and expression profile of two UDP-glucosyltransferases, UGT85K4 and UGT85K5, catalyzing the last step in cyanogenic glucoside biosynthesis in cassava. The Plant Journal 68(2):287-301 (2011)
Manihot esculenta (cassava) contains two cyanogenic glucosides, linamarin and lotaustralin, biosynthesized from l-valine and l-isoleucine, respectively. In this study, cDNAs encoding two uridine diphosphate glycosyltransferase (UGT) paralogs, assigned the names UGT85K4 and UGT85K5, have been isolated from cassava. The paralogs display 96% amino acid identity, and belong to a family containing cyanogenic glucoside-specific UGTs from Sorghum bicolor and Prunus dulcis. Recombinant UGT85K4 and UGT85K5 produced in Escherichia coli were able to glucosylate acetone cyanohydrin and 2-hydroxy-2-methylbutyronitrile, forming linamarin and lotaustralin. UGT85K4 and UGT85K5 show broad in vitro substrate specificity, as documented by their ability to glucosylate other hydroxynitriles, some flavonoids and simple alcohols. Immunolocalization studies indicated that UGT85K4 and UGT85K5 co-occur with CYP79D1/D2 and CYP71E7 paralogs, which catalyze earlier steps in cyanogenic glucoside synthesis in cassava. These enzymes are all found in mesophyll and xylem parenchyma cells in the first unfolded cassava leaf. In situ PCR showed that UGT85K4and UGT85K5 are co-expressed with CYP79D1 and both CYP71E7 paralogs in the cortex, xylem and phloem parenchyma, and in specific cells in the endodermis of the petiole of the first unfolded leaf. Based on the data obtained, UGT85K4 and UGT85K5 are concluded to be the UGTs catalyzing in planta synthesis of cyanogenic glucosides. The localization of the biosynthetic enzymes suggests that cyanogenic glucosides may play a role in both defense reactions and in fine-tuning nitrogen assimilation in cassava.
Damager, I., Engelsen, S.B., Blennow, A., Møller, B.L. and Motawia M.S. First principles insight into the a-glucan structures of starch: their synthesis, conformation and hydration. Chemical Reviews 110:2049-2080 (2010)
Jensen, K. and Møller, B.L. Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 71:132-141 (2010)
NADPH-cytochrome P450 oxidoreductase (CPR) serves as the electron donor to almost all eukaryotic cytochromes P450. It belongs to a small family of diflavin proteins and is built of cofactor binding domains with high structural homology to those of bacterial flavodoxins and to ferredoxin-NADP+ oxidoreductases. CPR shuttles electrons from NADPH through the FAD and FMN-cofactors into the central heme-group of the P450s. Mobile domains in CPR are essential for electron transfer between FAD and FMN and for P450 interaction. Blast searches identified 54 full-length gene sequences encoding CPR derived from a total of 35 different plant species. CPRs from vascular plants cluster into two major phylogenetic groups. Depending on the species, plants contain one, two or three paralogs of which one is inducible. The nature of the CPR–P450 interacting domains is well conserved as demonstrated by the ability of CPRs from different species or even from different kingdoms to at least partially complement each other functionally. This makes CPR an ideal bio-brick in synthetic biology approaches to re-design or develop entirely different combinations of existing biological systems to gain improved or completely altered functionalities based on the “share your parts” principle.
Laursen, T., Jensen K., and Møller, B.L. Conformational changes of the NADPH-dependent cytochrome P450 reductase in the course of electron transfer to cytochromes P450. BBA - Proteins & Proteomics 1814: 132-138 (2010)
The NADPH-dependent cytochrome P450 reductase (CPR) is a key electron donor to eucaryotic cytochromes P450 (CYPs). CPR shuttles electrons from NADPH through the FAD and FMN-coenzymes into the iron of the prosthetic heme-group of the CYP. In the course of these electron transfer reactions, CPR undergoes large conformational changes. This mini-review discusses the new evidence provided for such conformational changes involving a combination of a “swinging” and “rotating” model and highlights the molecular mechanisms by which formation of these conformations are controlled and thereby enables CPR to serve as an effective electron transferring “nano-machine”.
Møller, B.L. Functional diversifications of cyanogenic glucosides. Current Opinion in Plant Biology 13:337-346 (2010)
Cyanogenic glucosides are present in many plants and their ability to liberate toxic HCN offers an immediate chemical defense response to herbivores and pathogens causing damage of the plant tissue. Countermeasures have evolved to overcome this type of defense and in some cases herbivores and pathogens are able to exploit the presence of cyanogenic glucosides to their own advantage. In plants, cyanogenic glucosides have gained additional functionalities as transporters of nitrogen and operation of an endogenous turnover pathway may enable plants to withdraw the nitrogen and glucose deposited in cyanogenic glucosides for use in primary metabolism. The aim of this review is to provide an overview of the new knowledge on these diverse functionalities of cyanogenic glucosides.
Møller, B.L. Functioning dependent metabolons. Science 330:1328-1329 (2010)
“Our group has expertise in the physicochemical characterization of bio-nanostructures in bulk and at surfaces. Our interest goes over the relation structure-composition-function of biomembranes and in the structure-dynamics-function relation in membrane proteins.
Examples of research within the group include examinations of mechanistic aspects of nano-medicine uptake by cell membranes and the development of platforms for membrane protein studies. The use of nanodiscs gives us a strong advantage when studying membrane proteins. Each disc is a self-assembling particle composed of phospholipids and two amphipathic belt proteins. The nanodisc provides a native-like model of the cell membrane and each disc can harbor a single membrane protein. The structures of membrane proteins reconstituted in nanonodiscs are then elucidated using small angle X-ray and neutron scattering, while the formation of nanodisc films on surfaces allows us to use neutron and X-ray reflection and grazing X-ray diffraction to extract unique structural information for functional membrane proteins. The group has succeeded in probing the structure of membrane proteins in such nanodisc-loaded films by studying the conformation equilibrium of a plant reductase, the cytochrome P450 reductase (CPR). The group has also succeeded in determining how a 7-TM protein, the bacterio-rhodopsin, is oriented with respect to tilt-angle etc into the lipid bilayer of a nanodisc.”
- Marité Cárdenas Gómez, Chair of workgroup
Workgroup Participants
Marité Cárdenas Gómez, Chair of workgroup
Lise Arleth
Kell Mortensen
Robert Feidenhans'l
Nicholas Skar-Gislinge
Maria Wadsäter
Steen Laugesen Hansen
Workgroup Publications List
In this paper we present a systematic study of the morphology and composition of supported lipid bilayers (SLBs) formed by vesicle fusion using a wide variety of surface sensitive techniques that give information about the lateral as well as vertical structure and bilayer fluidity. SLBs of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) mixtures at five different bulk vesicle compositions were formed in such a way that the phase separation boundaries were crossed. For all compositions studied, the SLBs were systematically enriched with POPC compared to the nominal vesicle composition. Nevertheless, gel-fluid domain coexistence was observed for SLB compositions in which phase separation was expected based on the bulk phase diagram. The probable causes for the compositional difference in the SLBs are discussed in terms of the phase behaviour of the mixture and its effect on the membrane formation process by vesicle fusion.
Annaka, M., Mortensen, K., Vigild, M.E., Matsuura, T., Tsuji, S., Ueda, T., and Tsujinaka, H. Design of an Injectable in Situ Gelation Biomaterials for Vitreous Substitute. Biomacromolecules 12: 4011−4021 (2012)
To adapt the physical properties of living materials to their biological function, nature developed various types of polymers with outstanding physical behavior. One example is the vitreous body, which is important intraocular elements not only because of its optical and mechanical performances, but also due to its important role in the pathogenesis and treatment of conditions affecting adjacent tissues and eventually the whole eye. Here, we report a novel biocompatible material for injectable vitreous substitute, composed of thermosensitive amphiphilic polymer, which is capable of forming a transparent gel in the vitreous cavity. It is nontoxic, provides adequate support for the retina, and allows light to reach the sensory elements at the back of the eye. The amphiphilic polymer exhibits mechanical stability by assembling to form highly interconnected hydrophobic domains, which leads to the constitution of a network structure.
Gentile, L., Silva, B.F.B, Balog, S., Mortensen, K. and Olsson, U.
Structural transitions induced by shear flow and temperature variation in a nonionic surfactant/water system Journal of Colloid and Interface Science 372: 32–39 (2012)
In this study, we investigate structural transitions of tetraethylene glycol monohexadecyl ether (C16E4) in D2O as a function of shear flow and temperature. Via a combination of rheology, rheo-small-angle neutron scattering and rheo-small-angle light scattering, we probe the structural evolution of the system with respect to shear and temperature. Multi-lamellar vesicles, planar lamellae, and a sponge phase were found to compete as a function of shear rate and temperature, with the sponge phase involving the formation of a new transient lamellar phase with a larger spacing, coexisting with the preceding lamellar phase within a narrow temperature– time range. The shear flow behavior of C16E4 is also found to deviate from other nonionic surfactants with shorter alkyl chains (C10E3 and C12E4), resembling to the C16E7 case, of longer chain.
Andersen, H.D., Wang, C.H., Arleth, L., Peters, G.G. and Westh, P. Reconciliation of opposing views on membrane-sugar interactions. PNAS 108, 1874-1878 (2011)
It is well established that small sugars exert different types of stabilization of biomembranes both in vivo and in vitro. However, the essential question of whether sugars are bound to or expelled from membrane surfaces, i.e., the sign and size of the free energy of the interaction, remains unresolved, and this prevents a molecular understanding of the stabilizing mechanism. We have used small-angle neutron scattering and thermodynamic measurements to show that sugars may be either bound or expelled depending on the concentration of sugar. At low concentration, small sugars bind quite strongly to a lipid bilayer, and the accumulation of sugar at the interface makes the membrane thinner and laterally expanded. Above ∼0.2 M the sugars gradually become expelled from the membrane surface, and this repulsive mode of interaction counteracts membrane thinning. The dual nature of sugar–membrane interactions offers a reconciliation of conflicting views in earlier reports on sugar-induced modulations of membrane properties.
Hansen, S. The structure factor in small-angle scattering and the effect of deviation from spherical symmetry. J. Appl.Cryst. 44, 265-271 (2011)
The effect of deviation from spherical symmetry is studied for the structure factor. This is done by combining the analytical expression for the excluded volume of an ellipsoid of revolution with the expression for the excluded volume correlation function for a sphere. This approach makes it possible to estimate the effect of small deviations from spherical symmetry as a function of axial ratio and volume fraction for relatively low volume fractions. The calculations are relevant for the case of short-range potentials where the Percus-Yevick formula is frequently applied, and indicate that even minor deviations from spherical symmetry may lead to significant effects on the structure factor at low scattering angles.
Lafleur, J.P., Snakenborg, D., Nielsen. S.S., Møller, M., Toft, K.N., Menzel, A., Jacobsen, J.K., Vestergaard, B., Arleth, L. and Kutter, J.P. Automated microfluidic sample-preparation platform for high-throughput structural investigation of proteins by small-angle X-ray scattering. J Appl. Cryst. 44: 1090-1099 (2011).
A new microfluidic sample-preparation system is presented for the structural investigation of proteins using small-angle X-ray scattering (SAXS) at synchrotrons. The system includes hardware and software features for precise fluidic control, sample mixing by diffusion, automated X-ray exposure control, UV absorbance measurements and automated data analysis. As little as 15 µl of sample is required to perform a complete analysis cycle, including sample mixing, SAXS measurement, continuous UV absorbance measurements, and cleaning of the channels and X-ray cell with buffer. The complete analysis cycle can be performed in less than 3 min. Bovine serum albumin was used as a model protein to characterize the mixing efficiency and sample consumption of the system. The N2 fragment of an adaptor protein (p120-RasGAP) was used to demonstrate how the device can be used to survey the structural space of a protein by screening a wide set of conditions using high-throughput techniques.
Wadsäter, M., Simonsen, J.B., Lauridsen, T., Tveten, E., Naur, P., Bjørnholm, T., Wacklin, H., Mortensen, K., Arleth, L.,Feidenhans'l R., Cárdenas, M. Aligning Nanodiscs at the Air-Water Interface, A Neutron Reflection Study. Langmuir 27: 15065-150732011. (2011)
Nanodiscs are self-assembled nanostructures composed of a belt protein and a small patch of lipid bilayer, which can solubilize membrane proteins in a lipid bilayer environment. We present a method for the alignment of a well-defined two-dimensional layer of nanodiscs at the air–water interface by careful design of an insoluble surfactant monolayer at the surface. We used neutron reflectivity to demonstrate the feasibility of this approach and to elucidate the structure of the nanodisc layer. The proof of concept is hereby presented with the use of nanodiscs composed of a mixture of two different lipid (DMPC and DMPG) types to obtain a net overall negative charge of the nanodiscs. We find that the nanodisc layer has a thickness or 40.9 ± 2.6 Å with a surface coverage of 66 ± 4%. This layer is located about 15 Å below a cationic surfactant layer at the air–water interface. The high level of organization within the nanodiscs layer is reflected by a low interfacial roughness (4.5 Å) found. The use of the nanodisc as a biomimetic model of the cell membrane allows for studies of single membrane proteins isolated in a confined lipid environment. The 2D alignment of nanodiscs could therefore enable studies of high-density layers containing membrane proteins that, in contrast to membrane proteins reconstituted in a continuous lipid bilayer, remain isolated from influences of neighboring membrane proteins within the layer.
Castorph, S., Riedel, D., Arleth, L., Sztucki, M., Jahn, R., Holt, M. and Salditt, T.Structure Parameters of Synaptic Vesicles Quantified by Small-Angle X-Ray Scattering.Biophys. J. 98: 1200-1208 (2010)
Synaptic vesicles (SVs) are small, membrane-bound organelles that are found in the synaptic terminal of neurons, and which are crucial in neurotransmission. After a rise in internal [Ca2+] during neuronal stimulation, SVs fuse with the plasma membrane releasing their neurotransmitter content, which then signals neighboring neurons. SVs are subsequently recycled and refilled with neurotransmitter for further rounds of release. Recently, tremendous progress has been made in elucidating the molecular composition of SVs, as well as putative protein-protein interactions. However, what is lacking is an empirical description of SV structure at the supramolecular levelwhich is necessary to enable us to fully understand the processes of membrane fusion, retrieval, and recycling. Using small-angle x-ray scattering, we have directly investigated the size and structure of purified SVs. From this information, we deduced detailed size and density parameters for the protein layers responsible for SV function, as well as information about the lipid bilayer. To achieve a convincing model fit, a laterally anisotropic structure for the protein shell is needed, as a rotationally symmetric density profile does not explain the data. Not only does our model confirm many of the preexisting ideas concerning SV structure, but also for the first time, to our knowledge, it indicates structural refinements, such as the presence of protein microdomains.
Jensen, L.B., Mortensen, K., Pavan, G.N., Kasimova, M.R., Jensen, D.K., Gadzhyeva, V., Nielsen, H.M., and Foged, C. Molecular Characterization of the Interaction between siRNA and PAMAM G7 Dendrimers by SAXS, ITC, and Molecular Dynamics Simulations Biomacromolecules 11: 3571–3577 (2010)
A prerequisite for the use of dendrimers as drug delivery vehicles is the detailed molecular understanding of the drug interaction. The purpose of this study was to characterize the self-assembly process between siRNA and generation 7 poly(amidoamine) dendrimers and the resulting dendriplexes in aqueous solution using structural and calorimetric methods combined with molecular dynamics simulations. Complexes with a length scale of 150 nm showed a decreasing size with increasing amine-to-phosphate ratio by dynamic light scattering. At the molecular level, individual dendrimers studied by small-angle X-ray scattering (SAXS) showed no change in size upon siRNA binding, suggesting a rigid sphere behavior. Isothermal titration calorimetry (ITC) demonstrated exothermic binding with a concentration-dependent collapse of complexes. Both the experimentally determined ΔHbind and size were in close accordance with molecular dynamics simulations. This study demonstrates the unique complementarity of SAXS, ITC, and modeling for the detailed description of the molecular interactions between dendrimers and siRNA during dendriplex formation.
Skar-Gislinge, N., Bæk Simonsen J., Mortensen, K., Feidenhans’l, R., Sligar S.G., Møller, B.L., Bjørnholm, T. and Arleth L. 'Elliptical Structure of Phospholipid Bilayer Nanodiscs Encapsulated by Scaffold Proteins: Casting the Roles of the Lipids and the Protein'. JACS 132 (39): 13713–13722 (2010)
Phospholipid bilayers host and support the function of membrane proteins and may be stabilized in disc-like nanostructures, allowing for unprecedented solution studies of the assembly, structure, and function of membrane proteins (Bayburt et al. Nano Lett. 2002, 2, 853-856). Based on small-angle neutron scattering in combination with variable-temperature studies of synchrotron small-angle X-ray scattering on nanodiscs in solution, we show that the fundamental nanodisc unit, consisting of a lipid bilayer surrounded by amphiphilic scaffold proteins, possesses intrinsically an elliptical shape. The temperature dependence of the curvature of the nanodiscs prepared with two different phospholipid types (DLPC and POPC) shows that it is the scaffold protein that determines the overall elliptical shape and that the nanodiscs become more circular with increasing temperature. Our data also show that the hydrophobic bilayer thickness is, to a large extent, dictated by the scaffolding protein and adjusted to minimize the hydrophobic mismatch between protein and phospholipid. Our conclusions result from a new comprehensive and molecular-based model of the nanodisc structure and the use of this to analyze the experimental scattering profile from nanodiscs. The model paves the way for future detailed structural studies of functional membrane proteins encapsulated in nanodiscs.
Hoiberg-Nielsen, R., Westh, P. and Arleth, L. The Effect of Glycosylation on Interparticle Interactions and Dimensions of Native and Denatured Phytase. Biophys. J96: 153-161 (2009)
Glycosylation affects the physical properties of proteins in a number of ways including solubility and aggregation behavior. To elucidate the mechanism underlying these effects, we have measured second virial coefficients (A2) of the heavily glycosylated pheniophora lycii phytase (Phy) and its enzymatically deglycosylated counterpart (dgPhy) in native and in denatured form by means of small angle x-ray scattering. The measured A2-values show that the native forms of Phy and dgPhy are equally repulsive at the studied pH 8 where A2 equals 10.9 0.1 104 mL mol g2. However, when thermally denatured, the A2 of dgPhy decreases to 9.0 0.2 104 mL mol g2 whereas it remained unchanged for Phy. In accord with earlier investigations, the p(r)-function measured here suggested that the glycans did not affect the peptide structure of the native protein. Conversely, glycosylation markedly changed the structure of thermally denatured protein. This was evident from the radius of gyration, which increased by 32% for Phy and only 11% for dgPhy on denaturation. We suggest that this expanding effect of the glycans on the denatured protein conformation relies on steric hindrance that limits the range of torsion angles available to the polypeptide.
Kirkensgaard, J.J.K.and Hyde, S. Beyond amphiphiles: coarse-grained simulations of star-polyphile liquid crystalline assemblies. Phys. Chem. Chem. Phys. 11: 2016-2022 (2009)
We have simulated the self-assemblyof a novel class of three-arm molecules, ABC star-architecture polyphiles, using coarse-grained bead simulations. A number of topologically complex liquid crystalline mesostructures arise that can be related to the better-known bicontinuous mesophases of lyotropic amphiphilic systems. The simulations reveal 3Dself-assemblies whose structural variations follow those expected assuming a simple steric molecular packing model as a function of star polyphile splay and relative volumes of each arm in the polyphile. The splay of each arm, characterised by the 3D wedge-shape emanating from the core of each molecule to its exterior induces torsion of the interfaces along the triple lines, whereas differences in the relative volumes of arms induce curvature of the triple lines. Three distinct mesostructures are described, characterised by their micro-domain topologies, which are unknown in simpler amphiphilic systems, but resemble in some respects bicontinuous mesophases. These three- (or more) arm polyphilic systems offer an interesting extension to the better-knownself-assembly of (two-arm)amphiphiles in solution.
Kirkensgaard, J.J.K., Holm, J.K, Larsen, J.K. and Posselt, D. Simulation of small-angle X-ray scattering from thylakoid membranes. J. Appl. Cryst. 42 (2009)
Small-angle X-ray scattering (SAXS) patterns are calculated from a three-dimensional model of photosynthetic thylakoid membranes. The intricate structure of the thylakoids is represented by sampling random `electron density points' on geometric surfaces. The simulation setup works as a virtual instrument, allowing direct comparison with experimental data. The simulations qualitatively reproduce experimental data and thus clarify the structural origin of the scattering features. This is used to explain recent SAXS measurements and as a guideline for new experiments and future quantitative modeling. The setup has general applicability for model testing purposes when modeling scattering from membrane systems of complex geometries.
Knaapila, M., Svensson, C.S., Barauskas, J., Zackrisson, M., Nielsen, S.S., Nørgaard Toft, K., Vestergaard, B., Arleth, L., Olsson, U., Pedersen, J.S. and Cerenius, Y. A new small-angle X-ray scattering set-up on the crystallography beamline I711 at MAX-lab. J. Sunchrotron Radiation 16: 498-504, (2009)
A small-angle X-ray scattering (SAXS) set-up has recently been developed at beamline I711 at the MAX II storage ring in Lund (Sweden). An overview of the required modifications is presented here together with a number of application examples. The accessible q range in a SAXS experiment is 0.009-0.3 Å-1 for the standard set-up but depends on the sample-to-detector distance, detector offset, beamstop size and wavelength. The SAXS camera has been designed to have a low background and has three collinear slit sets for collimating the incident beam. The standard beam size is about 0.37 mm × 0.37 mm (full width at half-maximum) at the sample position, with a flux of 4 × 1010 photons s-1 and = 1.1 Å. The vacuum is of the order of 0.05 mbar in the unbroken beam path from the first slits until the exit window in front of the detector. A large sample chamber with a number of lead-throughs allows different sample environments to be mounted. This station is used for measurements on weakly scattering proteins in solutions and also for colloids, polymers and other nanoscale structures. A special application supported by the beamline is the effort to establish a micro-fluidic sample environment for structural analysis of samples that are only available in limited quantities. Overall, this work demonstrates how a cost-effective SAXS station can be constructed on a multipurpose beamline.
Nielsen, S.S., Nørgaard Toft, K., Snakenborg, D., Jeppesen, M.G., Jacobsen, J.K., Vestergaard, B., Kutter, J.P. and Arleth, L. BioXTAS RAW, a software program for high-throughput automated small-angle X-ray scattering data reduction and preliminary analysis. J. Appl. Cryst. 42: 959-964 (2009)
A fully open source software program for automated two-dimensional and one-dimensional data reduction and preliminary analysis of isotropic small-angle X-ray scattering (SAXS) data is presented. The program is freely distributed, following the open-source philosophy, and does not rely on any commercial software packages. BioXTAS RAW is a fully automated program that, via an online feature, reads raw two-dimensional SAXS detector output files and processes and plots data as the data files are created during measurement sessions. The software handles all steps in the data reduction. This includes mask creation, radial averaging, error bar calculation, artifact removal, normalization and q calibration. Further data reduction such as background subtraction and absolute intensity scaling is fast and easy via the graphical user interface. BioXTAS RAW also provides preliminary analysis of one-dimensional data in terms of the indirect Fourier transform using the objective Bayesian approach to obtain the pair-distance distribution function, PDDF, and is thereby a free and open-source alternative to existing PDDF estimation software. Apart from the TIFF input format, the program also accepts ASCII-format input files and is currently compatible with one-dimensional data files from SAXS beamlines at a number of synchrotron facilities. BioXTAS RAW is written in Python with C++ extensions.
Szewczykowski, P., Andersen, K., Schulte, L., Mortensen, K., Vigild, M.E., and Ndoni, S. Elastomers with Reversible Nanoporosity. Macromolecules, 42: 5636-5641, (2009)
An elastomer was created via cross-linking a diene block of a polyisoprene −polydimethylsiloxane (PI−PDMS) block copolymer in the ordered state of hexagonal morphology, followed by the quantitative removal of the PDMS component. The elastomer material collapsed following etching of the PDMS and apparently showed no resulting nanoporosity or structure resembling the precursor morphology in contrast to similar polydiene-based nanoporous material. However, the collapsed elastomer displayed surprising properties when exposed to a solvent. In the gel state the material recovers the original nanostructure and displays liquid-filled cavities. Upon several cycles of swelling and drying the cavities open and close in a reversible fashion. When exposed to a nonsolvent, the material remains collapsed. This discriminating behavior of liquid-material interaction holds potential for the use of these materials in advanced separation or load-release systems
Mortensen, K. and Vigild, M.E. Reinvestigation of the modulated lamellar structure in diblock copolymers. Macromolecules, 42:1685–1690, (2009)
We report extended crystallographic studies on shear-aligned block copolymer systems within the metastable modulated lamellae (ML) state. With studies limited to the “classical” orientations parallel and perpendicular to shear plane, the apparent modulated state would likely have been assigned simple lamellar. Surprisingly, upon rotating the sample to intermediate angles additional scattering reflections appear, which reveal the apparent ML phase much beyond what was expected. The modulated structure is a slightly distorted fcc structure. With the sample sheared at relatively low temperature, presumably below the stable gyroid phase, we find a very well resolved ML texture corresponding to a simple twin structure of the distorted fcc structure.
When shear-aligned within the hexagonal cylinder phase, and quenced to the gyroid phase or slightly below, we find ML alignment into a two-dimensional powder texture.
Høiberg-Nielsen, R., Westh, P., Skov, L.K. and Arleth, L. Interrelationship of Steric Stabilization and Self-Crowding of a Glycosylated Protein. Biophys. J. 97: 1445-1453 (2009)
In the eukaryotic cell, protein glycosylation takes place in the crowded environment of the endoplasmatic reticulum. With the purpose of elucidating the impact of high concentration on the interactions of glycoproteins, we have conducted a series of small-angle x-ray scattering experiments on the heavily glycosylated enzyme Peniophora lyciiphytase (Phy) and its deglycosylated counterpart (dgPhy). The small-angle x-ray scattering data were analyzed using an individual numerical form factor for each of the two glycoforms combined with two structure factors, a hard sphere and a screened coulomb potential structure factor, respectively, as determined by ab initio analysis. Based on this data analysis, three main conclusions could be drawn. First, at comparable protein concentrations (mg/ml), the relative excluded volume of Phy was ∼75% higher than that of dgPhy, showing that the glycans significantly increase excluded-volume interactions. Second, the relative excluded volume of dgPhy increased with concentration, as expected; however, the opposite effect was observed for Phy, where the relative excluded volume decreased in response to increasing protein concentration. Third, a clear difference in the effect of salinity on the excluded-volume interactions was observed between the two glycol forms. Although the relative excluded volume of dgPhy decreased with increasing ionic strength, the relative excluded volume of Phy was basically insensitive to increased salinity. We suggest that protrusion forces from the glycans contribute to steric stabilization of the protein, and that glycosylation helps to sustain repulsive electrostatic interactions under crowded conditions. In combination, this aids in stabilizing high concentrations of glycosylated proteins.