Tomas Laursen PhD defense
Factors stimulating metabolon formation
- From single molecules to macromolecular assemblies
Time: February 20th, 13:00
Place: Auditorium A1-05.01
Reception: Room M117-1
Summary
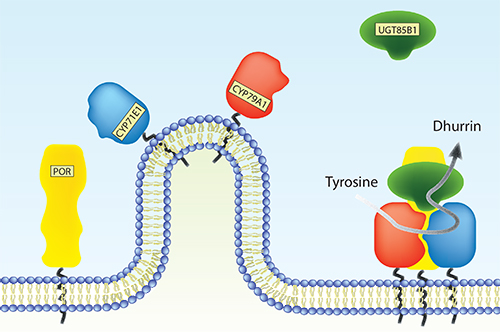
Plants produce an immense number of bio-active natural products for defense, attraction and signaling. Many of these compounds have potential uses as pharmaceutical chemicals, flavors and colorants as well as being targets for metabolic engineering (Part 7, research paper I). The cytochrome P450 (CYP) enzyme superfamily plays a key role in the formation of the diversity of these compounds. Microsomal CYPs receive electrons from the NADPH-dependent cytochrome P450 oxidoreductase (POR) as reducing equivalents necessary for their monooxygenase activity. Many natural product biosynthetic pathways include multiple CYP isoforms, which are thought to organize in multienzyme complexes, metabolons, on the endoplasmic reticulum surface. It is essential to discover the factors that stimulate assembly of metabolons in order to understand the complexity of metabolic flux.
This study has focused on the biosynthetic pathway of dhurrin, a defense compound found in the subtropical crop-plant Sorghum bicolor. Biosynthesis of dhurrin involves two CYP isoforms (CYP79A1 and CYP71E1), POR and a soluble glycosyltransferase (UGT85B1). During catalysis, these enzymes are thought to form a metabolon where the substrate, tyrosine, is channeled towards dhurrin. This serves to increase the flux and prevent leakage of labile and toxic intermediates. The role of membrane environment, protein-protein interactions and putative additional components such as scaffold proteins and other redox proteins on dhurrin metabolon formation has so far been elusive.
A variety of bio-mimetic membrane systems were used to study structural (Part 7, research paper II) and functional properties of individual enzymes of the dhurrin pathway down to the single molecule level (Part 7, research paper V) and assembly of the entire metabolon (Part 3.3). The functional properties of POR were found to be critically dependent on membrane reconstitution. The activity of both CYP79A1 and CYP71E1 reconstituted in liposomes were stimulated by native Sorghum lipid compared to a simple lipid composition. Furthermore, the soluble UGT85B1 were found to stimulate assembly of the membrane bound dhurrin enzymes i.e. POR, CYP79A1 and CYP71E1.
The ability of a styrene maleic acid (SMA) co-polymer to extract discrete lipid particles (SMALPs) with associated proteins and their interaction partners were employed to isolate the dhurrin pathway by a single purification step (Part 4.1). Upon affinity purification of POR, we were able to co-purify CYP79A1 and CYP71E1, which were quantified within the eight most abundant proteins. This technology has a huge potential for identification of unknown interaction partners and even entire membrane bound biosynthetic pathways.
The results obtained in this Ph.D study provide unique insight in the mechanisms stimulating metabolon formation involved in natural product biosynthesis. Future studies will build on the platforms established and hopefully provide further insight in regulatory mechanisms governing the assembly of dynamic metabolons.
Supervisors
Professor Birger Lindberg Møller
Assistant Professor Nikos S Hatzakis
Opponents
Dr. Daniele Werck, Universite de Strasbourg
Senior Research Scientist Ilia Denisov, University of Illinois
Details
Time: February 20th, 2014, 13:00-17:00
Place: Auditorium A1-05.01, Thorvaldsensvej 40, 1871 Frederiksberg C
Reception: M117-1
Contact
 Tomas Laursen
Tomas Laursen
tola@plen.ku.dk
+45-35333329
