SEMINAR: Unravelling the mechanisms of P450s
- from human to bacterial
With Professor Rita Bernhardt from University of Saarlandes, Germany, and Dr. Ilia Denisov from University of Illinois, US
Talk by Professor, Dr. Rita Bernhardt

Professor Dr. Rita Bernhardt, Institut für Biochemie, Universität des Saarlandes, Germany, bernhardt.biochem.uni-sb.de/
Title: Terpene hydroxylation with bacterial cytochromes P450
Abstract: Cytochromes P450 are the first enzymes to modify terpene synthase products. Although major advances concerning the engineering of plant P450s have been made, these enzymes still pose great challenges, especially in terms of microbial expression. Therefore, soluble bacterial P450s catalyzing terpene hydroxylation came into the focus of interest for biotechnological production of terpenoid compounds thus demonstrating the potential of these P450 enzymes for the production of terpenoids for the fragrance, flavor and pharmaceutical industry.
We have demonstrated highly selective hydroxylations of di- and triterpenes such as resin acids and 11-keto-β-boswellic acid by members of the CYP106 family of Bacillus megaterium and by CYP105 from Streptomyces griseolus. The 3D structure of CYP106A2 with abietic acid has been resolved and the structural basis for highly selective substrate hydroxylation has been explained. Furthermore, a set of terpene hydroxylases was identified among myxobacterial P450s and the production of (+)-eremohilene  as a novel biotechnological product was demonstrated. Moreover, highly selective hydroxylations of α and β-ionone as well as nootkatone and other terpenes have been obtained using different members of myxobacterial P450s thus presenting a valuable toolbox for the production of terpenoids. Taken together, bacterial P450s are promising tools for the production of modified terpenes.
as a novel biotechnological product was demonstrated. Moreover, highly selective hydroxylations of α and β-ionone as well as nootkatone and other terpenes have been obtained using different members of myxobacterial P450s thus presenting a valuable toolbox for the production of terpenoids. Taken together, bacterial P450s are promising tools for the production of modified terpenes.
Talk by Dr. Ilia Denisov

Dr. Ilia Denisov, senior research scientist, University of Illinois, US, Dept. of Biochemisty, sligarlab.life.uiuc.edu
/index.html
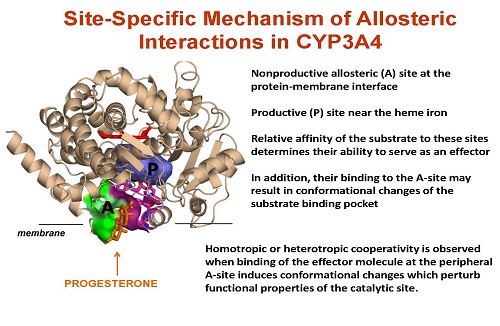
Title: Site-specific mechanism of allosteric interactions in human cytochrome P450 CYP3A4
Abstract: CYP3A4 is an important drug-metabolizing enzyme in the human liver, metabolizing a large fraction of the marketed drugs. It has a large active site that can simultaneously bind multiple substrates as well as a peripheral binding site located at the protein-membrane interface, which contributes to the allosteric properties of the enzyme.
Recently we demonstrated that the binding of progesterone (PGS) at this allosteric site activates carbamazepine (CBZ) epoxidation, and suggested that this was due to a specific conformational change involving movement of Phe213 when PGS binds to the allosteric site. This movement changes the shape and size of the substrate binding pocket and improves productive binding of CBZ (Biochemistry (2015) 54, 2227-2239). In order to explore this allosteric mechanism, we probed the role of the Phe-213 side chain by generating three mutants: F213Y, F213S, and F213A. Functional complex of CYP3A4 and its redox partner, cytochrome P450 reductase (CPR), was assembled in Nanodiscs containing a POPC bilayer. Product formation, NADPH oxidation and spectral titration experiments were performed using both PGS and CBZ as substrates. The considerable changes in activity of all three mutants indicate an essential role of F213 in the overall functional properties of CYP3A4.