Synbio and intellectual property rights: 6 recommendations
On 26th November 2013, the Danish Agency for Science, Technology and Innovation organized an expert meeting on “Synthetic Biology & Intellectual Property Rights” in Copenhagen sponsored by the European Research Area Network in Synthetic Biology (ERASynBio). The meeting brought together ten experts from different countries with a variety of professional backgrounds to discuss emerging challenges and opportunities at the interface of synthetic biology and intellectual property rights. The aim of this article is to provide a summary of the major issues and recommendations discussed during the meeting.
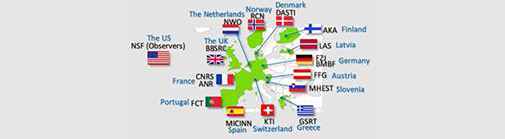
The ERASynBio consortium consists of 16 governmental funding bodies from 12 EU Member States (Austria, Denmark, Finland, France, Germany, Greece, Latvia, Netherlands, Portugal, Spain, Slovenia, and UK) and two Associated Countries (Norway and Switzerland)
RECOMMENDATION 1 – Empirical Evidence and Open Source Software Tools
Develop cheap, easy-to-use open source software tools to map the patent landscape and provide FTO analysis tailored to the particular needs of the SB community in order to properly determine the actual impact of IPRs on SB.
RECOMMENDATION 2 – Use of Public Domain Tools
Encourage and enable scientists to employ tools unencumbered by IPRs for developing foundational technologies in SB
RECOMMENDATION 3 – Patent Quality and Transparency
Establish collaborations between the SB community, patent offices and other government agencies to improve the quality of issued patents and increase transparency of patent ownership.
RECOMMENDATION 4 – Best Licensing Practices
Adopt and promote guidelines and best practices in licensing SB inventions for foundational technologies.
RECOMMENDATION 5 – Private Ordering Mechanisms
Explore opportunities for private ordering mechanisms to improve transparency of ownership and facilitate licensing
RECOMMENDATION 6 – Legislative and Regulatory Changes
Explore the need/options for further legislative and regulatory changes in order to facilitate R&D and innovation in SB, while at the same time protecting the enforceability of well-defined patent claims on the use of genetically encoded functions and concrete applications in industry, health care and agriculture
 Associate Professor Timo Minssen is member of the Center for Synthetic Biology Steering Group. His research evolves around intellectual property law and innovation law and how it applies in relation to synthetic biology
Associate Professor Timo Minssen is member of the Center for Synthetic Biology Steering Group. His research evolves around intellectual property law and innovation law and how it applies in relation to synthetic biology
